At first glance, the dip in FDA approvals suggests a quiet year, but the data reveals a landscape transforming at breakneck speed. 2025 wasn't about volume, it was about validating high-barrier science, from breaking 20-year deadlocks in pain management to engineering entirely new biologic scaffolds.
Five Key Takeaways:
The Numbers Tell One Story, The Science Tells Another!
Three years of decline. But look closer at what those 46 approvals represent:
A year of firsts for Adnectin-based biologic, non-profit gene therapy, DPP1 inhibitor, NaV1.8 sodium channel blocker, drugs ever approved for bronchiectasis and IgA nephropathy.
The industry isn't producing fewer innovations. It's producing harder ones.
Each first-in-class approval required navigating uncharted regulatory territory, developing novel biomarkers, and integrating complex multi-omic datasets to prove mechanisms with no clinical precedent.
Oncology Leads the Path (16 approvals, 35%):
Cancer still leads, but it's how these drugs work that matters:
New ADC targets: TROP2 and c-Met expand beyond HER2 breast cancer.
The 10th bispecific T-cell engager: Linvoseltamab for multiple myeloma
First FAK inhibitor: Defactinib in novel-novel combination with MEK inhibitor
First ClpP activator: Dordaviprone for aggressive brain tumors
The Rare Disease Revolution:
Nephrology: Two first-ever drugs for IgA nephropathy (sibeprenlimab and atrasentan)
Pulmonology: Brensocatib became the first drug for non-cystic fibrosis bronchiectasis, treating 500,000 Americans who previously had only physical therapy. Forecast: $6.3B peak sales.
Hereditary Angioedema: Three drugs targeting different pathway nodes approved in one year
Gene Therapy: Etuvetidigene autotemcel, the first non-profit gene therapy for Wiskott-Aldrich syndrome, treating <10 patients/year in the US
Other Notable Areas:
Cardiology (5 approvals): First cardiac myosin inhibitor, first Adnectin-based biologic (lerodalcibep for lipid-lowering)
Pain Management: Suzetrigine, the first NaV1.8 inhibitor, non-opioid painkiller after 20+ years of failures ($3.7B forecast)
Infectious Disease: Two novel antibiotic scaffolds (gepotidacin, zoliflodacin) fighting antimicrobial resistance
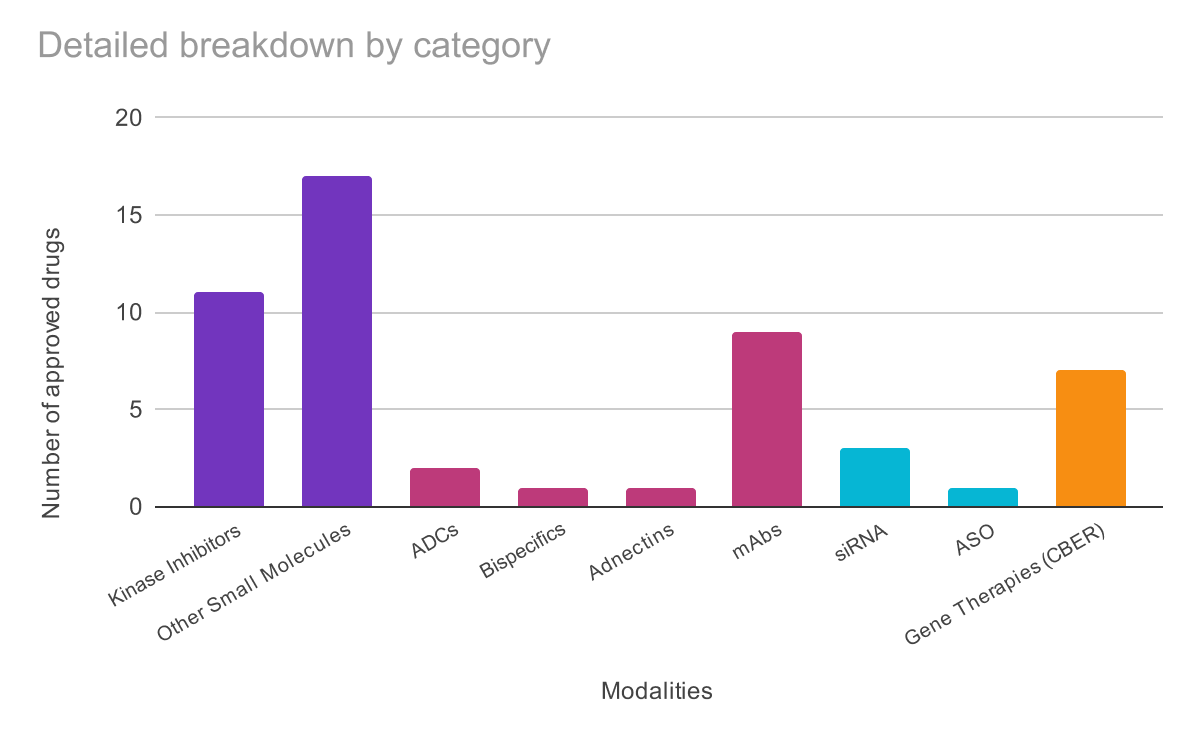
Small Molecules: 28 approvals (61%); Still dominant, but increasingly sophisticated:
11 kinase inhibitors including remibrutinib (the 100th kinase inhibitor milestone)
First-in-class mechanisms: NaV1.8 blocker, DPP1 inhibitor, ClpP activator, novel antibiotic scaffolds
Biologics: 13 approvals (28%)
2 ADCs with novel targets (TROP2, c-Met)
First Adnectin: Lerodalcibep validates fibronectin-based scaffolds that are smaller, more stable than antibodies
Bispecifics: BCMA × CD3 T-cell engager
Oligonucleotides: 3 approvals (7%)
2 siRNA therapies
1 antisense oligonucleotide
Cell & Gene Therapies: 8 CBER approvals
First non-profit gene therapy
First cell-sheet gene therapy
First immunotherapy for HPV-driven respiratory disease
The pattern: Drug developers aren't choosing between platforms, rather they're mastering multiple modalities simultaneously.
1. Lerodalcibep: Platform Validation
The first Adnectin-based biologic isn't just another PCSK9 inhibitor. It validates an entirely new class of therapeutics built from fibronectin domains instead of antibody frameworks, offering smaller size, better stability (room-temperature storage), and simpler manufacturing. If this works, it unlocks targets where traditional antibodies face size or stability constraints.
2. Suzetrigine: Persistence Pays Off
After 20+ years of failed sodium channel inhibitors, Vertex's NaV1.8 blocker finally delivers a non-opioid painkiller. This validates an "undruggable" target and proves selectivity between closely related ion channels is achievable. The $3.7B forecast reflects both massive unmet need and the challenge of competing with cheap generics while proving efficacy in chronic pain.
3. Etuvetidigene Autotemcel: The Non-Profit Model
Fondazione Telethon's gene therapy broke the mold with the first non-profit approved therapy treating <10 US patients annually, economically unviable for traditional pharma. If this model succeeds, it becomes the blueprint for dozens of ultra-rare gene therapies.
Here's the uncomfortable truth: Validating these approvals require data infrastructure that most organizations don't have.
Consider what proving suzetrigine's efficacy demanded:
For datopotamab deruxtecan (TROP2 ADC):
Each required connecting genomic, proteomic, structural, clinical, and literature data scattered across systems. When data lives in silos, every decision requires manual integration, creating bottlenecks when speed matters most.
The 2025 winners weren't just those with the best science. They were those who could connect data to answer complex questions fastest.
The 43 complete response letters are equally instructive:
Manufacturing killed science: Scholar Rock's apitegromab and Regeneron's odronextamab both rejected due to third-party fill-finish facility issues.
Trial design matters: Replimune's oncolytic virus couldn't isolate virus effects from PD-1 blocker, thus historical controls deemed inadequate.
Endpoints are critical: Stealth's elamipretide rejected in May, approved in September after resubmission with different surrogate endpoint.
The lesson: Even validated mechanisms fail without execution in manufacturing, trial design, and regulatory strategy, areas where integrated data identifies risks before they become rejections.
As molecules get smarter like Adnectins, PROTACs, oligonucleotides, gene therapies, your data infrastructure needs to get smarter too.
What Polly Does:
Connects fragmented data: Public databases (TCGA, GTEx) + proprietary datasets, genomics + proteomics + clinical outcomes in unified workspace
Accelerates first-in-class discovery: Mine novel targets like NaV1.8 or ClpP, model mechanism plausibility, benchmark against competitors in real-time
De-risks development: Design trials using historical control databases, select optimal biomarkers from multi-omic analysis, predict safety from tissue profiling
Why Now?
The 2025 approvals prove the industry is tackling:
First-in-class mechanisms with no precedent
Novel modalities requiring new paradigms
Rare diseases where traditional economics don't apply
When apitegromab failed due to manufacturing, it wasn't a science problem. When vusolimogene's historical controls were inadequate, it was a data design problem.
46 approvals doesn't mean innovation is slowing, it simply means innovation is maturing.
The pharmaceutical industry shifted from "how many drugs" to "how transformative are they?"
More first-in-class mechanisms
Greater modality diversity
Expansion into untreatable diseases
New business models for ultra-rare conditions
The question isn't whether innovation is declining.
It's whether your data infrastructure can keep pace.
Ready to accelerate your next breakthrough? Connect with Elucidata to learn how Polly transforms complex biomedical data into competitive advantage.